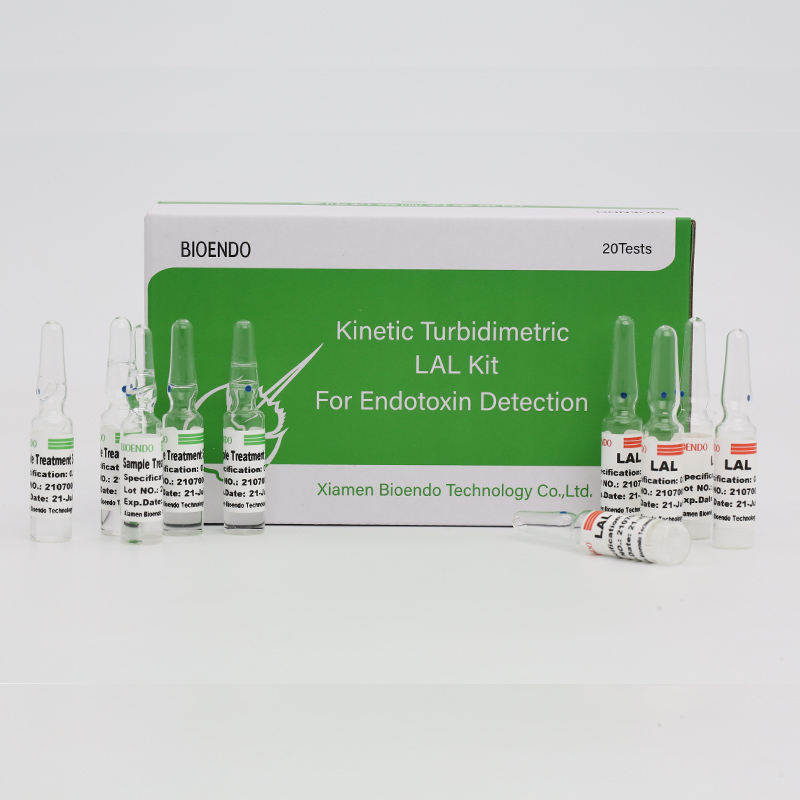
Endotoxin Assay Kit ya Plasma ya Anthu
Endotoxin Assay Kitkwa Plasma ya Anthu
1. Zambiri Zogulitsa
CFDA yachotsedwaClinical diagnostic Endotoxin assay kitamayesa kuchuluka kwa endotoxin mu plasma yaumunthu.Endotoxin ndi gawo lalikulu la khoma la cell la mabakiteriya a Gram Negative ndipo ndiye mkhalapakati wofunikira kwambiri wa sepsis.Kuchuluka kwa endotoxin nthawi zambiri kungayambitse kutentha thupi, kusintha kwa kuchuluka kwa maselo oyera amwazi, nthawi zina, kugwedezeka kwamtima.Zimachokera ku factor Cpathway mu limulus Polyphemus (magazi a nkhanu ya akavalo).Ndi kinetic microplate reader ndi endotoxin assay software, Endotoxin assay kit imazindikira kuchuluka kwa endotoxin mu plasma yamunthu pasanathe ola limodzi.Chidacho chimabwera ndi plasma pre-treatment reagent yomwe imachotsa zoletsa mu plasma panthawi ya endotoxin assay.
2. Product Parameter
Mtundu woyeserera: 0.01-10 EU/ml
3. Mawonekedwe a Zamalonda ndi Kugwiritsa Ntchito
Amabwera ndi plasma pretreatment solutions, amachotsa zinthu zolepheretsa mu plasma ya munthu.
Zindikirani:
Lyophilized Amebocyte Lysate (LAL) reagent yopangidwa ndi Bioendo amapangidwa kuchokera ku amebocyte lysate opangidwa ndi magazi a nkhanu.
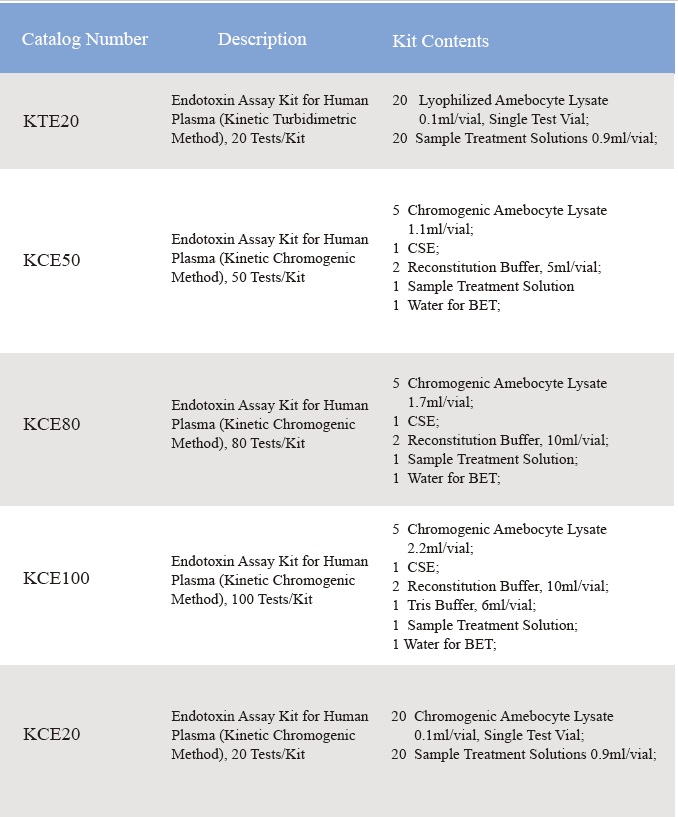
Kumva kwa Lyophilized Amebocyte Lysate ndi mphamvu ya Control Standard Endotoxin imayesedwa motsutsana ndi USP Reference Standard Endotoxin.Zida za Lyophilized Amebocyte Lysate reagent zimabwera ndi malangizo azinthu, Certificate of Analysis, MSDS.