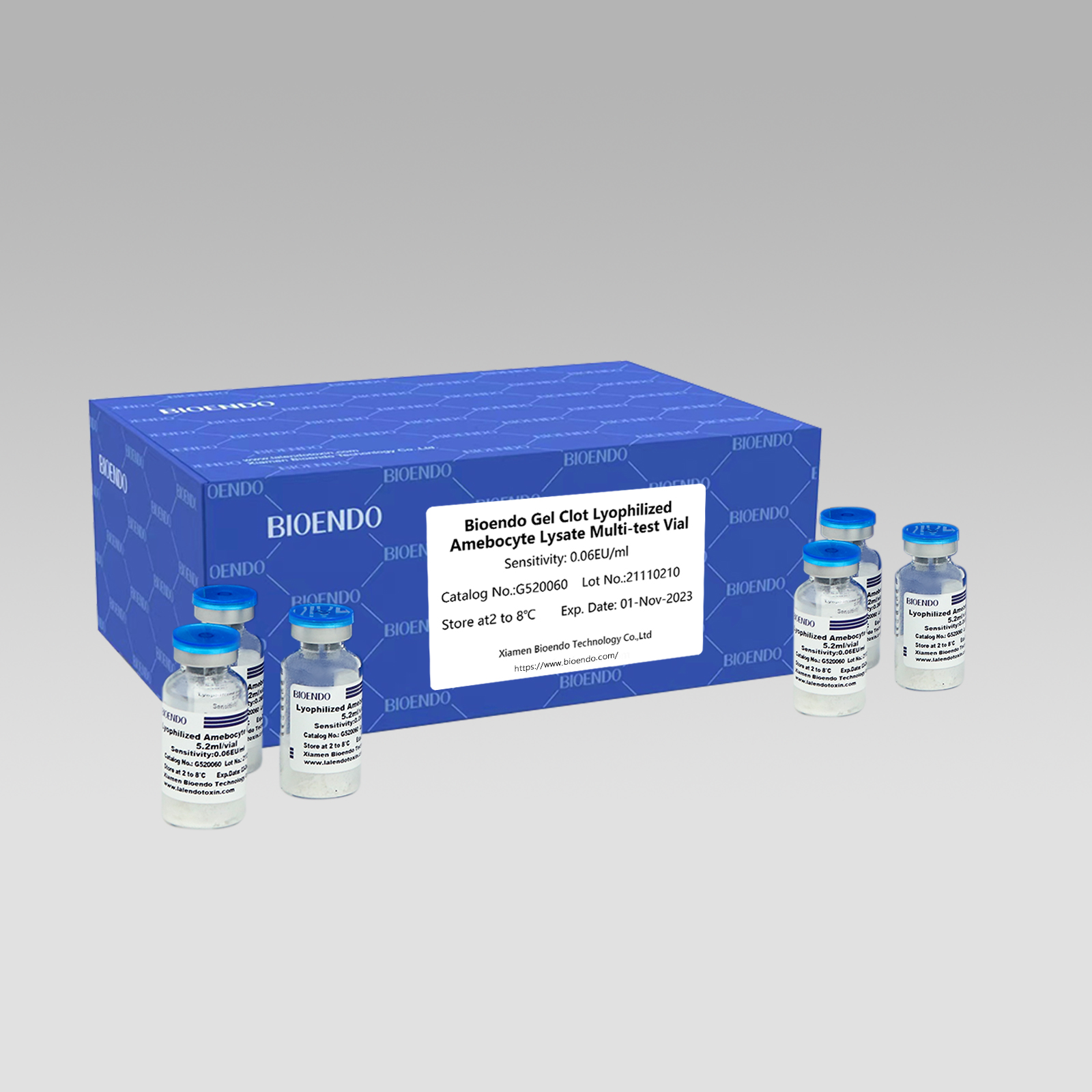
Gel Clot Lyophilized Amebocyte Lysate Multi-test Vial G52
Bioendo G52 mndandanda makamaka ntchito kuyesera ntchitokuyesa kwa bakiteriya endotoxinmonga njira ya Bioassay.
1. Zambiri Zogulitsa
Njira ya Gel Clot Lyophilized Amebocyte Lysate Multi-test Vial ndi Lyophilized Amebocyte Lysate reagent yomwe imasankha ndikugwiritsa ntchito njira ya gel clot kuti izindikire endotoxin kapena pyrogen.
Monga njira yofala, kuyesa kwa gel-clot kwa endotoxin ndikosavuta ndipo sikufuna chida chapadera komanso chokwera mtengo.Bioendo imapereka Gel Clot Lyophilized Amebocyte Lysate - LAL reagent mu 5.2ml pa vial.
2. Zopangira Zamankhwala
Kumverera kosiyanasiyana: 0.03EU/ml, 0.06EU/ml, 0.125EU/ml, 0.25EU/ml, 0.5 EU/ml
3. Kugwiritsa Ntchito Mankhwala
Kuyenerera kwa endotoxin (pyrogen), madzi a jakisonikuyesa kwa endotoxin, zopangirakuyesa kwa endotoxinkapena kuwunika kwa endotoxin panthawi yopangira makampani opanga mankhwala kapena opanga zida zamankhwala.
Zindikirani:
Lyophilized Amebocyte Lysate (LAL reagent) yopangidwa ndi Bioendo imapangidwa kuchokera ku lysate ya amebocytes (maselo oyera a magazi) kuchokera ku nkhanu ya horseshoe.
Reagent yapaderayi yakhala chida chofunikira m'makampani opanga mankhwala ndi zida zamankhwala kuti azindikire ma endotoxins a bakiteriya.Amebocyte a nkhanu ya horseshoe ali ndi chinthu chotchedwa Lyophilized Amebocyte Lysate, chomwe chimagwirizana ndi ma endotoxins a bakiteriya popanga jekeseni ngati gel.Izi ndiye maziko a mayeso a LAL, omwe amagwiritsidwa ntchito kuonetsetsa chitetezo cha zida zamankhwala, mankhwala, ndi zinthu zina zomwe zimalumikizana ndi thupi la munthu.
Kugwiritsa ntchito LAL reagent kwasintha njira yakuzindikira kwa endotoxinm'munda wa zamankhwala kuposa kuyesa kwa Kalulu.Kukhudzika kwake kosayerekezeka ndi kutsimikizika kwake kumapangitsa kuti ikhale gawo lofunikira pakuwongolera komanso kutsimikizira chitetezo chamankhwala, biologics, ndi zida zamankhwala.Mayeso a LAL ndi njira yachangu komanso yodalirikakuzindikira kwa endotoxin, kupereka zotsatira mu mphindi 60 zokha.Kuchita bwino kumeneku kumapangitsa kuti pakhale zisankho zachangu komanso zolondola pankhani yotulutsa zinthu, ndikumawonjezera chitetezo chokwanira komanso mphamvu yamankhwala ndi zida.
Bioendo's Lyophilized Amebocyte Lysate (LAL reagent) imapangidwa pansi pamiyezo yolimba kuti iwonetsetse kuti ikugwira ntchito komanso yodalirika.Kampaniyo yadzipereka kugwiritsa ntchito njira zokhazikika pakukolola nkhanu za akavalo kuti achepetse vuto lililonse pagulu lawo.Poika patsogolo zamoyo wa zolengedwa izi, Bioendo amaonetsetsa kuti gwero lamtengo wapatalili lipitilizidwa popanga ma reagents a LAL.Kuonjezera apo, kufufuza kosalekeza ndi kuyesetsa kwachitukuko kumayang'ana kwambiri kupititsa patsogolo ntchito ndi kusinthasintha kwaLAL kuyesa endotoxin, kupititsa patsogolo ntchito yawo m’mafakitale a zamankhwala ndi mankhwala.
Njira yothetsera gel osakanizaChithunzi cha LAL, reconstituted lysate reagent amapeza mayeso osachepera 50 pa vial:
| Nambala ya Catalog | Sensitivity (EU/ml kapena IU/ml) | ml/botolo | Mayesero/Vial | Mbale/Paketi |
| G520030 | 0.03 | 5.2 | 50 | 10 |
| G520060 | 0.06 | 5.2 | 50 | 10 |
| G520125 | 0.125 | 5.2 | 50 | 10 |
| G520250 | 0.25 | 5.2 | 50 | 10 |
| G520500 | 0.5 | 5.2 | 50 | 10 |
Zogulitsa:
The Lyophilized Amebocyte Lysate - LAL reagent sensitivity ndi Control Standard Endotoxin potency amayesedwa motsutsana ndi USP Reference Standard Endotoxin.Zida za Lyophilized Amebocyte reagent zimabwera ndi malangizo azinthu, Certificate of Analysis, MSDS.
Kodi pali kusiyana kotani pakati pa Bioendo single test vial ndi angapo test vial?
● Mayeso amodzi: yambitsaninso imodzilimulus lysate testkapena kuyitanalimulus amebocytendi BET madzi mu vial galasi kapena galasi ampoule.
● Mipikisano mayeso: reconstitute ndi lysate reagent ndi BET madzi, ndiyeno kuwonjezera chizindikiro kuchuluka kwa lysate reagent kutsatira COA kwa anachita chubu kapena bwino mbale ntchito.Palibe kusiyana kwa chitsanzo chisanadze ndondomeko;malinga ndi kuchuluka kwa kuyezetsa komwe kumagwiritsidwa ntchito, kukula kwachitsanzo komwe kumagwiritsidwa ntchito poyesa kamodzi ndikokulirapo kuposa kukula kwachitsanzo komwe kumagwiritsidwa ntchito poyesa zingapo.
Chifukwa chiyani zida za gel clot assay G52 ndizopadera za kuchuluka kwa zitsanzo?
1. Multi test LAL reagent kuti azindikire endotoxin pogwiritsira ntchito zitsanzo zambiri za LAL assay operation process.
2. G52 mndandanda wa Gel clot endotoxin assay multi test glass vial palibe chifukwa chowerengera microplate chapamwamba.Mu kuyesa kwa LAL njira yake yopangira ma incubation ndi madzi osamba kapena chofungatira chowuma chowuma ndi chipangizo chosavuta.
3. Ubwino wapamwamba wa endotoxin wopanda chubu (<0.005EU/ml) ndi Ubwino wapamwamba wa nsonga zaulere za pyrogen (<0.005EU/ml) monga zogwiritsidwa ntchito zotsimikiziridwa kuti zitsimikizire zotsatira zolondola.
4. Kusankha Bioendo single LAL test vial kapena multi LAL test vial ndi kuchuluka kwa zitsanzo, chandamale ndiMayeso a LAL a pyrogenskuzindikira.
Zogwirizana ndi endotoxin test assay:
Madzi a Bacterial Endotoxins Test (BET), Ndikulimbikitsani TRW50 kapena TRW100
Endotoxin wopanda galasi chubu ( dilution chubu ), Ndibwino kuti T1310018 ndi T107540
Malangizo a Pyrogen aulere, Ndikulimbikitsani PT25096 kapena PT100096
Pipettor, ndikulimbikitsani PSB0220
Test Tube Rack
Incubation Instrument (Kusamba kwa Madzi kapena Dry Heat Incubator ), kupangira Bioendo Dry Heat Incubator TAL-M2 ndi mabowo 60 amodzi modula.
Vortex Mixter, Ndikulimbikitsani VXH.
Control Standard Endotoxin, CSE10V.